how to analyze uv absorptions from graph with multiple peaks|interpretation of uv spectra : supermarket So, if we start to measure the UV spectra of a bunch of compounds, we start to see evidence of that conjugation phenomenon from the indigo and carotene. Each time we add a double bond to a conjugated system, the wavelength of light . Fala aí rapaziada! Bem-vindos eu ao canal Tudo de PLS, eu trago downloads de mod's, patchs e kits, espero que gostem. Bem-vindo! INSCREVA-SE e 🔔:https://y.
{plog:ftitle_list}
14 de fev. de 2024 · Gruta Azul Club, #20 entre Porto Alegre clubes: 191 avaliações e 24 fotos detalhadas. Encontrar no mapa e ligar para reservar uma mesa. Entrar. English . R. Gaspar Martins, 230, Porto Alegre, Rio Grande do Sul, Brasil, 90220-160 . .
Where UV-vis spectroscopy becomes useful to most organic and biological chemists is in the study of molecules with conjugated pi systems. In these groups, the energy gap for π -π* transitions is smaller than for isolated double bonds, .
We have been talking in general terms about how molecules absorb UV and visible light – now let's look at some actual examples of data from a UV-vis absorbance .The diagram below shows a simple UV-visible absorption spectrum for buta-1,3-diene - a molecule we will talk more about later. Absorbance (on the vertical axis) is just a measure of the amount of light absorbed. The higher the value, the .So, if we start to measure the UV spectra of a bunch of compounds, we start to see evidence of that conjugation phenomenon from the indigo and carotene. Each time we add a double bond to a conjugated system, the wavelength of light .This page takes a brief look at how UV-visible absorption spectra can be used to help identify compounds and to measure the concentrations of coloured solutions. It assumes that you know how these spectra arise, and know what is meant .
Polyaromatic systems have rather complicated UV spectra, but as each additional aromatic ring is added, a shift of about 30 nm occurs in the absorption maximum of the most prominent peak. If naphthalene has λ max at 220 nm, where .
IR Spectroscopy Practice Problems. By itself, Infrared (IR) spectroscopy isn’t a great technique for solving the structure of an unknown molecule.However, we’ve seen that IR spectroscopy can a great technique for .If the isoprene spectrum on the right was obtained from a dilute hexane solution (c = 4 * 10-5 moles per liter) in a 1 cm sample cuvette, a simple calculation using the above formula indicates a molar absorptivity of 20,000 at the maximum .
влагомер нефти удвн 1пм
uv visible absorption spectrum
It is therefore relative easy to identify the aldehyde group (together with the C=O stretching at about 1700 cm-1) since essentially no other absorptions occur at these wavenumbers (see the example of IR spectrum of butanal in Figure 6.4d ). The stretching vibration of triple bonds C≡C and C≡N have absorption bands of about 2100~2200 cm-1 . 4.Broad Peaks: May indicate a complex mixture or a compound with multiple overlapping absorptions, causing the peak to widen. 5.Peak Intensity: 6.Strong Peaks: Suggest a high concentration of the corresponding chemical bond or functional group. 7.Weak Peaks: Indicate a lower concentration of the chemical bond or functional group.You can move between the peaks lines: select spectrum of interest (see Available spectra fig.) and click on the spectrum diagram, use the left/right keyboard cursor to move between the peaks. The current peak will be selected with the peak cursor (see fig.) directly on the diagram and in the Calculated Spectrum peaks list (see fig.).What absorptions would the following compounds have in an IR spectra? Answer. 1. 2. A) A OH peak will be present around 3300 cm-1 for methanol and will be absent in the ether. B) 1-pentene will have a alkene peak around 1650 cm-1 for the C=C and there will be another peak around 3100 cm-1 for the sp 2 C-H group on the alkene
UV-Vis spectroscopy is used to quantify the amount of DNA or protein in a sample, for water analysis, and as a detector for many types of chromatography. Kinetics of chemical reactions are also measured with UV-Vis spectroscopy by taking repeated UV-Vis measurements over time. UV-Vis measurements are generally taken with a spectrophotometer.This peak is not terribly useful, as just about every organic molecule that you will have occasion to analyze has these bonds. Nevertheless, it can serve as a familiar reference point to orient yourself in a spectrum. You will notice that there are many additional peaks in this spectrum in the longer-wavelength 400 -1400 cm-1 region.
The whole idea of UV spectroscopy is that different compounds might absorb photons of different wavelengths based on their electronic structures. We might be able to look at the UV spectrum of a compound and tell its identity or structure; that task would be especially straightforward if we had a few different options to choose from.is used for band/peak position throughout, and this is expressed in the commonly used units of wavenum-ber (cm 1). The average modern infrared instrument records spectra from an upper limit of around 4000cm 1 (by convention) down to 400cm 1 as defined by the optics of the instrument (commonly based on potassium bromide, KBr).
The frequency scale at the bottom of the chart is given in units of reciprocal centimeters (cm-1) rather than Hz, because the numbers are more manageable. The reciprocal centimeter is the number of wave cycles in one centimeter; whereas, frequency in cycles per second or Hz is equal to the number of wave cycles in 3*10 10 cm (the distance covered by light in one second). I am working on a project in my lab, and we are analyzing capsaicin using HPLC. I am trying to find an appropriate wavelength for the HPLC using a UV-Vis spectrometer. When analyzing the pure capsaicin standard dissolved in methanol, I of course use methanol as a blank. However, I got two peaks, one around 230nm and one at 280nm.
A molecule can be identified by comparing its absorption peak to a data bank of spectra. IR spectroscopy is very useful in the identification and structure analysis of a variety of substances, including both organic and inorganic compounds. It can also be used for both qualitative and quantitative analysis of complex mixtures of similar compounds. Most UV-vis instruments can analyze solid samples or suspensions with a diffraction apparatus (Figure \(\PageIndex{7}\)), but this is not common. UV-vis instruments generally analyze liquids and solutions most . Effect of wavelength selection on the linearity of a Beer’s law plot. Another reason for measuring absorbance at the top of an absorbance peak is that it provides for a more sensitive analysis. Note that the green Beer’s law .
Understanding UV-Vis Spectroscopy Will Make You More Fun At Parties. In today’s post we’ll discuss why most molecules are colourless, introduce the useful technique of UV-visible spectroscopy, and finally explain . For instance, if you're analyzing a fruit sample for anthocyanins, you might see multiple peaks. Which peak is cyanidin-3-glucoside, pelargonidin-3-glucoside, or another anthrocyanin? By comparing the UV-Vis spectrum of that peak to reference spectra, you can make a more definitive identification. Consider the Challenges The UV/Vis spectrum for cranberry juice in Figure \(\PageIndex{5}\) shows a single broad band for the anthocyanin dyes that are responsible for its red color. The IR spectrum for ethanol in Figure \(\PageIndex{6}\) shows multiple .A molecule has a variety of covalent bonds, and each bond has different vibration modes, so the IR spectrum of a compound usually shows multiple absorption bands. The horizontal axis indicates the position of an absorption band, but instead of using frequency to show the absorbed radiation, wavenumbers ( , in the unit of cm -1 ) are used as a .
signal-to-noise ratio of the spectrum and can assist in the analysis of weakly absorbing samples. The spectral resolution is user-defined and helps to distinguish closely spaced absorption peaks, and is expressed in wavenumbers. The simplified correlation table on the right allows users to extract structural information from IR spectra.analysis, UV-Vis chemometric analysis, azo dyes, dilution, high absorbance Abstract The new Thermo Scientific ™ NanoDrop QC Software for the Thermo Scientific ™ NanoDrop Onec Microvolume UV-Vis Spectrophotometer allows scientists to perform chemometric analysis of high absorbance chemical samples in real-time without the need to dilute. Functional Groups Containing the C=O Bond. Ketones have IR absorptions associated with the C=O bond. Below is a spectrum of 2-butanone. Notable peak: the strong band at 1712 cm-1 for the C=O.; Note: for conjugated ketones, the carbonyl peak will shift 20-30 cm-1 lower.; Aldehydes have IR absorptions associated with the C=O bond and the aldehyldic proton.
The spectra were recorded using a UV–vis–NIR spectrophotometer (UV-3600 Shimadzu) equipped with a 15 cm integrating sphere in the spectral range 250–800 nm. Each time the sample holder was rotated to a different position (by ∼45°). Barium sulfate (BaSO 4, Riedel-de Haen) was used to dilute the samples (1:100) and was used as a reference. A OH peak will be present around 3300 cm-1 for methanol and will be absent in the ether. 1-pentene will have a alkene peak around 1650 cm-1 for the C=C and there will be another peak around 3100 cm-1 for the sp 2 C-H group on the .Beckman DU640 UV/Vis spectrophotometer. Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry [1] [2] [3] refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. [2] Being relatively inexpensive and easily implemented, this methodology is widely .
Ultraviolet-visible (UV-Vis) spectroscopy is a widely used technique in many areas of science ranging from bacterial culturing, drug identification and nucleic acid purity checks and quantitation, to quality control in the beverage industry and chemical research. This article will describe how UV-Vis spectroscopy works, how to analyze the output data, the technique's .
влагомер опилок фауна пм
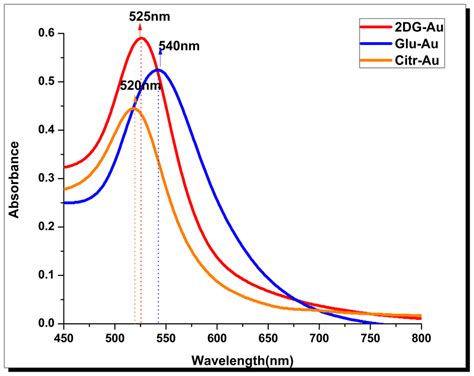
uv absorption spectrum diagram
влагомер почвы для комнатных растений
uv absorption spectra example
$10.99
how to analyze uv absorptions from graph with multiple peaks|interpretation of uv spectra